This week’s Innovation Partners BioBlog reports on the accelerated timeline for Anthem’s new PBM as well as GlaxoSmithKline’s move to acquire Merck KGaA cancer immunotherapy. ASCO names treatments for rare cancer as the “Advance of the Year,” and the University of Pittsburgh School of Medicine’s discovery may repurpose an anti-rejection drug as a cancer treatment. This and more in the Innovation Partners BioBlog.
The Out-of-Pocket Cost Burden for Specialty Drugs in Medicare Part D in 2019
High out-of-pocket costs continue to challenge Medicare Part D recipients, according to a new report. Specialty tier drugs, or those costing more than $670 per month, are of special concern to Part D enrollees. Drugs included in this category are often those used to treat chronic conditions such as multiple sclerosis, rheumatoid arthritis, hepatitis C, as well drugs used to treat cancer. The sobering findings from this report indicate that Medicare Part D enrollees not receiving low income subsidiaries can expect to pay thousands of dollars per year in out-of-pocket costs.
Read More
ASCO Names Advance of the Year: Progress in Treating Rare Cancers
The American Society of Clinical Oncology (ASCO) named “Progress in Treating Rare Cancers” as the Advance of the Year thanks in part to the many new treatment options available to patients with rare, difficult-to-treat cancers. Some of the advances cited by ASCO include treatment options for desmoid tumors; anaplastic thyroid carcinoma (ATC); foregut, midgut, and hindgut neuroendocrine tumors; uterine serous carcinoma; and tenosynovial giant cell tumor.
Read More
Payer Roundup—Moody’s: Anthem’s accelerated PBM launch bad for Cigna
Anthem’s plans to accelerate launch of its in-house pharmacy benefits manager, IngenioRX, appears to have Cigna on the edge. Moody’s Investor Services stated that the aggressive timeline, which moved the launch of IngenioRX from 2020 to 2019, is credit positive. It will reduce cash flow to Cigna—which now owns Anthem’s previous PBM, Express Scripts—by $750 million to $800 million in 2019.
Read More
Senate bill backed by patient groups proposes value-based ‘annuity’ payments for pricey treatments
Two U.S. senators, Bill Cassidy, M.D. (R-La.) and Mark Warner (D-Va.) recently introduced the Patient Affordability, Value and Efficiency Act. The act proposes amending the Social Security Act so that it will be legal for drug and medical device companies to strike payment deals with insurers that are linked to the performance of their products. This “pay for performance” model is similar to payment models currently in use with expensive therapies such as gene therapy and cell therapies. Fourteen patient advocacy groups, including Michael J. Fox’s foundation, support the proposed act.
Read More
Trump administration to eliminate safe harbors for drug rebates to PBMs
The Trump administration wants to eliminate safe harbor protections for drug rebates negotiated by pharmacy benefit managers and instead offer those protections to discounts passed directly to consumers. The proposed changes is under review with the Office of Management and Budgeting. The Administrator believes it will improve transparency in the pharmacy supply chain.
Read More
Half a million fewer deaths from lung cancer in 40 years
Cancer Research UK announced good news this week: lung cancer deaths among men are down nearly 60% over the past 40 years. The discovery last century that smoking caused lung cancer and the push to decrease the number of smokers has helped reduce the cancer rate. Additionally, more people have access to treatment, including surgery, which likely reduced the mortality rate. Lung cancer remains the most common form of cancer; 19,300 men and around 16,300 women still die from lung cancer every year
Read More
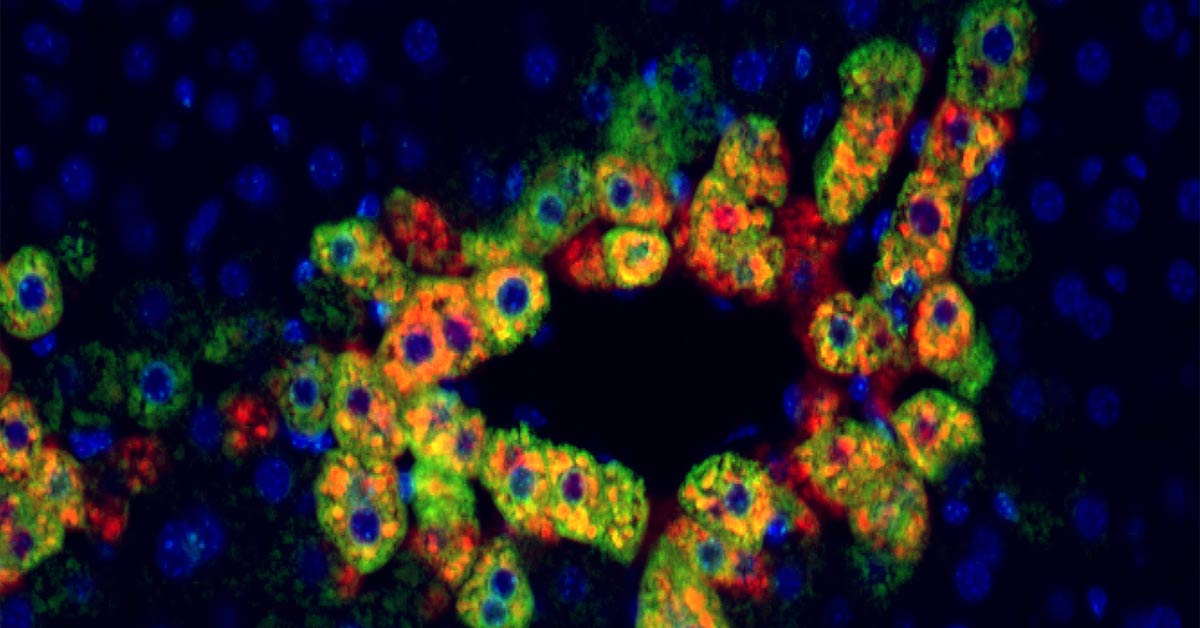
Anti-Rejection Drug Could be Repurposed to Treat Cancer
Research from the University of Pittsburgh School of Medicine in animal models and patient tissues has identified a new molecular pathway in the liver that suggests a commonly used anti-rejection medication could be repurposed to treat certain liver cancers. Researchers found that liver cancers with a specific mutation in the β-catenin gene are possibly more susceptible to rapamycin, a commonly used anti-rejection medication in transplantation. Approximately 20 to 35 percent of liver cancers have a β-catenin mutation, but there is little understanding of how and why these mutations could be aiding the growth of cancerous cells.
Read More
GSK pays up to $4.2 billion for Merck KGaA cancer immunotherapy
GlaxoSmithKline agreed to pay $4.2 billion for Merck KGaA cancer immunotherapy rights. Merck will receive upfront payment for M7824 (bintrafusp alfa) with additional payment possible depending on development milestones in lung cancer. Merck will continue to book U.S. sales from the product while GSK can book revenues from sales from the rest of the world. This move from GSK is the latest in its march towards adding more cancer drugs to its portfolio.
Read More



